Meteoric ¹⁰Be/⁹Be in Carbonates
Meteoric 10Be, produced in the atmosphere, can be used to quantify denudation rates (the sum of weathering and erosion) when normalized with the stable trace element 9Be. We measure this 10Be/9Be ratio in river sediments, soils, but also in water or even plants.
In this project, we have developed this new method for carbonate rock. We have chosen the Jura Mountains in the NE of France as a natural “laboratory” because runoff data and water chemistry are well known here, with which our new method can be compared.
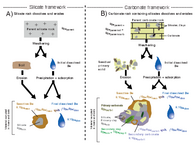
Carbonate systems are more complex than the weathering of silicate rocks (e.g. basalt, Fig. 1A) because various new phases (e.g. secondary carbonates, Fig. 1B) can form by precipitation from the water, but these have nothing to do with weathering. Such precipitation is controlled by supersaturation of the water with Ca2+ cations, which are washed out of the rock. As soon as CO2 (e.g. bound to roots in soils or at springs from air) is available, CaCO3 (calcium carbonate) precipitates. At springs, the precipitation product is called “travertine”.
To validate our new method, we quantified secondary carbonate formation using 13C/12C (d13C). By measuring d13C on rock, sediment, soil and, above all, water, we can calculate what proportion comes from the rock (i.e. primarily formed calcium carbonate) and how much secondary carbonate has precipitated from the water. We have also calculated how much 9Be comes from silicate clays. Such silicate phases can be found in every carbonate rock.
Our results show that despite this complexity, the new 10Be/9Be proxy gives very similar denudation rates for soils to those from solute loads in rivers calculated from water chemistry measurements. A direct comparison shows that soils erode at about 300 t/km2*yr, but rates measured from sediment at springs are somewhat higher. We assume that subsurface weathering also occurs as these sediments are channeled through the karst system. Weathering rates from water chemistry, however, could only reflect the weathering in soils, because Ca2+ concentrations are set there.
Project Time Frame
- since 2017